what best explains whether bromine (br) or neon (ne) is more likely to form a covalent bond?
Bonding & Molecular Structure |
|---|
Structure & Bonding
The written report of organic chemistry must at some bespeak extend to the molecular level, for the physical and chemic properties of a substance are ultimately explained in terms of the structure and bonding of molecules. This module introduces some basic facts and principles that are needed for a discussion of organic molecules.
Electronic Configurations |
|---|
Electron Configurations in the Periodic Table
|
| ||||||||||||||||||||||||||||||||||||||||||
Four elements, hydrogen, carbon, oxygen and nitrogen, are the major components of most organic compounds. Consequently, our understanding of organic chemistry must accept, as a foundation, an appreciation of the electronic structure and properties of these elements. The truncated periodic tabular array shown in a higher place provides the orbital electronic structure for the first 18 elements (hydrogen through argon). Co-ordinate to the Aufbau principle, the electrons of an cantlet occupy quantum levels or orbitals starting from the everyman energy level, and proceeding to the highest, with each orbital holding a maximum of ii paired electrons (opposite spins).

Electron shell #1 has the lowest energy and its s-orbital is the starting time to be filled. Vanquish #ii has four higher energy orbitals, the 2s-orbital being lower in energy than the iii 2p-orbitals. (x, y & z). As we progress from lithium (atomic number=3) to neon (diminutive number=10) across the second row or period of the tabular array, all these atoms start with a filled 1s-orbital, and the 2s-orbital is occupied with an electron pair before the 2p-orbitals are filled. In the 3rd catamenia of the table, the atoms all have a neon-similar cadre of 10 electrons, and beat #iii is occupied progressively with eight electrons, starting with the 3s-orbital. The highest occupied electron beat is called the valence crush, and the electrons occupying this beat out are chosen valence electrons.
The chemic properties of the elements reflect their electron configurations. For example, helium, neon and argon are exceptionally stable and unreactive monoatomic gases. Helium is unique since its valence trounce consists of a single south-orbital. The other members of grouping viii have a feature valence shell electron octet (nsii + npx 2 + npy 2 + npz 2). This group of inert (or noble) gases also includes krypton (Kr: 4s2, 4phalf-dozen), xenon (Xe: 5s2, 5psix) and radon (Rn: 6s2, 6p6). In the periodic table in a higher place these elements are colored beige.
The halogens (F, Cl, Br etc.) are ane electron short of a valence shell octet, and are amid the almost reactive of the elements (they are colored red in this periodic table). In their chemical reactions element of group vii atoms reach a valence shell octet by capturing or borrowing the eighth electron from another cantlet or molecule. The alkali metals Li, Na, Thousand etc. (colored violet higher up) are also exceptionally reactive, but for the reverse reason. These atoms have just one electron in the valence shell, and on losing this electron arrive at the lower shell valence octet. Equally a consequence of this electron loss, these elements are normally encountered as cations (positively charged atoms).
The elements in groups 2 through 7 all exhibit characteristic reactivities and bonding patterns that tin in large part be rationalized by their electron configurations. It should be noted that hydrogen is unique. Its location in the periodic table should not propose a kinship to the chemistry of the alkali metals, and its office in the structure and properties of organic compounds is unlike that of any other chemical element.
Bonding & Valence |
|---|
Chemical Bonding and Valence
Every bit noted earlier, the inert gas elements of group viii exist every bit monoatomic gases, and do non in general react with other elements. In contrast, other gaseous elements exist every bit diatomic molecules (H2, Nii, Oii, F2 & Cl2), and all but nitrogen are quite reactive. Some dramatic examples of this reactivity are shown in the following equations.
| 2Na + Clii |  | 2NaCl |
| 2H2 + O2 |  | 2H2O |
| C + O2 |  | CO2 |
| C + 2Fii |  | CF4 |
Why do the atoms of many elements interact with each other and with other elements to give stable molecules? In addressing this question it is instructive to begin with a very uncomplicated model for the attraction or bonding of atoms to each other, then progress to more sophisticated explanations.
Ionic Bonding
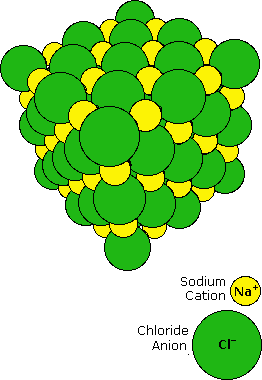 When sodium is burned in a chlorine atmosphere, it produces the compound sodium chloride. This has a high melting point (800 ºC) and dissolves in water to to give a conducting solution. Sodium chloride is an ionic compound, and the crystalline solid has the structure shown on the right. Transfer of the lone 3s electron of a sodium atom to the half-filled 3p orbital of a chlorine atom generates a sodium cation (neon valence beat) and a chloride anion (argon valence trounce). Electrostatic allure results in these oppositely charged ions packing together in a lattice. The attractive forces property the ions in place can exist referred to as ionic bonds.
When sodium is burned in a chlorine atmosphere, it produces the compound sodium chloride. This has a high melting point (800 ºC) and dissolves in water to to give a conducting solution. Sodium chloride is an ionic compound, and the crystalline solid has the structure shown on the right. Transfer of the lone 3s electron of a sodium atom to the half-filled 3p orbital of a chlorine atom generates a sodium cation (neon valence beat) and a chloride anion (argon valence trounce). Electrostatic allure results in these oppositely charged ions packing together in a lattice. The attractive forces property the ions in place can exist referred to as ionic bonds.
By clicking on the NaCl diagram, a model of this crystal will be displayed and may be manipulated.
Covalent Bonding
The other three reactions shown to a higher place give products that are very dissimilar from sodium chloride. Water is a liquid at room temperature; carbon dioxide and carbon tetrafluoride are gases. None of these compounds is equanimous of ions. A different bonny interaction betwixt atoms, called covalent bonding, is involved hither. Covalent bonding occurs by a sharing of valence electrons, rather than an outright electron transfer. Similarities in concrete backdrop (they are all gases) advise that the diatomic elements H2, N2, Otwo, Fii & Cltwo also take covalent bonds.
Examples of covalent bonding shown below include hydrogen, fluorine, carbon dioxide and carbon tetrafluoride. These illustrations use a elementary Bohr notation, with valence electrons designated by colored dots. Annotation that in the first case both hydrogen atoms achieve a helium-like pair of 1s-electrons by sharing. In the other examples carbon, oxygen and fluorine achieve neon-like valence octets past a similar sharing of electron pairs. Carbon dioxide is notable considering it is a case in which two pairs of electrons (four in all) are shared past the same two atoms. This is an example of a double covalent bail.

These electron sharing diagrams (Lewis formulas) are a useful kickoff footstep in agreement covalent bonding, but it is quicker and easier to describe Couper-Kekulé formulas in which each shared electron pair is represented past a line between the atom symbols. Non-bonding valence electrons are shown every bit dots. These formulas are derived from the graphic notations suggested by A. Couper and A. Kekulé, and are not identical to their original drawings. Some examples of such structural formulas are given in the following tabular array.
| Common Proper noun | Molecular Formula | Lewis Formula | Kekulé Formula |
|---|---|---|---|
| Methane | CH 4 | 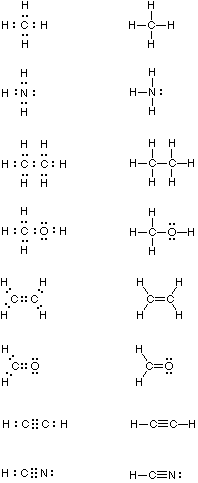 | |
| Ammonia | NH 3 | ||
| Ethane | C 2 H 6 | ||
| Methyl Alcohol | CH 4 O | ||
| Ethylene | C 2 H 4 | ||
| Formaldehyde | CH ii O | ||
| Acetylene | C two H two | ||
| Hydrogen Cyanide | CHN | ||
Multiple bonding, the sharing of two or more electron pairs, is illustrated by ethylene and formaldehyde (each has a double bond), and acetylene and hydrogen cyanide (each with a triple bond). Boron compounds such equally BH3 and BF3 are exceptional in that conventional covalent bonding does not aggrandize the valence beat out occupancy of boron to an octet. Consequently, these compounds accept an affinity for electrons, and they showroom exceptional reactivity when compared with the compounds shown above.
Valence
The number of valence shell electrons an cantlet must gain or lose to achieve a valence octet is called valence. In covalent compounds the number of bonds which are characteristically formed by a given cantlet is equal to that atom's valence. From the formulas written higher up, we make it at the following full general valence assignments:
| Atom | H | C | N | O | F | Cl | Br | I |
| Valence | ane | 4 | 3 | 2 | i | 1 | 1 | 1 |
The valences noted here stand for the nearly common form these elements assume in organic compounds. Many elements, such as chlorine, bromine and iodine, are known to be in several valence states in different inorganic compounds.
Accuse Distribution |
|---|
Charge Distribution
If the electron pairs in covalent bonds were donated and shared absolutely evenly there would be no stock-still local charges within a molecule. Although this is true for diatomic elements such every bit H2, Due north2 and O2, nearly covalent compounds show some caste of local charge separation, resulting in bond and / or molecular dipoles. A dipole exists when the centers of positive and negative charge distribution do not coincide.
Formal Charges
A large local charge separation usually results when a shared electron pair is donated unilaterally. The three Kekulé formulas shown hither illustrate this condition.
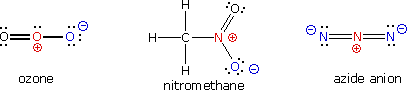
In the formula for ozone the fundamental oxygen atom has 3 bonds and a total positive accuse while the right mitt oxygen has a single bond and is negatively charged. The overall charge of the ozone molecule is therefore zero. Similarly, nitromethane has a positive-charged nitrogen and a negative-charged oxygen, the total molecular charge again being aught. Finally, azide anion has two negative-charged nitrogens and i positive-charged nitrogen, the total accuse being minus i.
In general, for covalently bonded atoms having valence shell electron octets, if the number of covalent bonds to an atom is greater than its normal valence it volition bear a positive charge. If the number of covalent bonds to an cantlet is less than its normal valence it volition carry a negative charge. The formal accuse on an atom may also be calculated past the post-obit formula:

Polar Covalent Bonds
| H 2.20 | Electronegativity Values for Some Elements | |||||
|---|---|---|---|---|---|---|
| Li 0.98 | Be ane.57 | B 2.04 | C two.55 | N 3.04 | O 3.44 | F 3.98 |
| Na 0.xc | Mg one.31 | Al 1.61 | Si 1.90 | P ii.xix | S 2.58 | Cl 3.16 |
| Thou 0.82 | Ca ane.00 | Ga 1.81 | Ge 2.01 | Every bit 2.18 | Se 2.55 | Br two.96 |
When two different atoms are bonded covalently, the shared electrons are attracted to the more electronegative atom of the bond, resulting in a shift of electron density toward the more electronegative atom. Such a covalent bond is polar, and will have a dipole (ane end is positive and the other stop negative). The degree of polarity and the magnitude of the bond dipole will exist proportional to the difference in electronegativity of the bonded atoms. Thus a O–H bond is more than polar than a C–H bond, with the hydrogen atom of the former beingness more than positive than the hydrogen bonded to carbon. Likewise, C–Cl and C–Li bonds are both polar, but the carbon end is positive in the erstwhile and negative in the latter. The dipolar nature of these bonds is oftentimes indicated past a partial charge notation (δ+/–) or by an pointer pointing to the negative end of the bail.

Although there is a modest electronegativity difference between carbon and hydrogen, the C–H bond is regarded as weakly polar at best, and hydrocarbons in full general are considered to exist non-polar compounds.
The shift of electron density in a covalent bond toward the more electronegative cantlet or group can exist observed in several ways. For bonds to hydrogen, acidity is ane criterion. If the bonding electron pair moves away from the hydrogen nucleus the proton volition be more easily transfered to a base (it volition exist more acidic). A comparing of the acidities of methyl hydride, water and hydrofluoric acid is instructive. Methane is essentially non-acidic, since the C–H bond is about non-polar. Equally noted above, the O–H bail of water is polar, and information technology is at least 25 powers of ten more acidic than methane. H–F is over 12 powers of x more acidic than water as a consequence of the greater electronegativity difference in its atoms.
Electronegativity differences may be transmitted through connecting covalent bonds by an inductive event. Replacing one of the hydrogens of water by a more electronegative atom increases the acidity of the remaining O–H bond. Thus hydrogen peroxide, HO–O–H, is 10 1000 times more acidic than h2o, and hypochlorous acid, Cl–O–H is ane hundred million times more acidic. This anterior transfer of polarity tapers off as the number of transmitting bonds increases, and the presence of more one highly electronegative cantlet has a cumulative result. For example, trifluoro ethanol, CF3CHtwo–O–H is about ten grand times more acidic than ethanol, CH3CH2–O–H.
Excellent physical evidence for the inductive effect is establish in the influence of electronegative atoms on the nmr chemical shifts of nearby hydrogen atoms.
Do Issues |
| |
|---|
![]()
Functional Groups |
|---|
Functional Groups
Functional groups are atoms or small groups of atoms (two to four) that exhibit a feature reactivity when treated with sure reagents. A particular functional grouping will nearly always display its characteristic chemic behavior when information technology is nowadays in a compound. Considering of their importance in understanding organic chemical science, functional groups take characteristic names that often carry over in the naming of individual compounds incorporating specific groups. In the following table the atoms of each functional group are colored blood-red and the characteristic IUPAC nomenclature suffix that denotes some (but not all) functional groups is also colored.
Functional Group Tables
Exclusively Carbon Functional Groups
| Group Formula | Class Proper noun | Specific Example | IUPAC Name | Common Proper name |
|---|---|---|---|---|
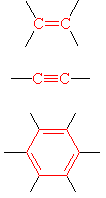 | Alkene | H2C=CHtwo | Ethene | Ethylene |
| Alkyne | HC≡CH | Ethyne | Acetylene | |
| Arene | CsixHhalf dozen | Benzene | Benzene |
Functional Groups with Unmarried Bonds to Heteroatoms
| Group Formula | Class Name | Specific Example | IUPAC Name | Common Name |
|---|---|---|---|---|
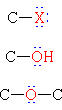 | Halide | HiiiC-I | Iodomethane | Methyl iodide |
| Booze | CHthreeCH2OH | Ethanol | Ethyl booze | |
| Ether | CH3CH2OCHtwoCH3 | Diethyl ether | Ether | |
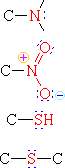 | Amine | H3C-NH2 | Aminomethane | Methylamine |
| Nitro Compound | HthreeC-NO2 | Nitromethane | ||
| Thiol | H3C-SH | Methanethiol | Methyl mercaptan | |
| Sulfide | HthreeC-S-CH3 | Dimethyl sulfide |
Functional Groups with Multiple Bonds to Heteroatoms
| Group Formula | Class Name | Specific Instance | IUPAC Proper name | Common Proper noun |
|---|---|---|---|---|
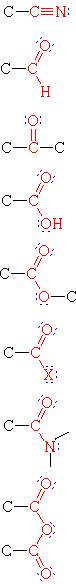 | Nitrile | HiiiC-CN | Ethanenitrile | Acetonitrile |
| Aldehyde | HthreeCCHO | Ethanal | Acetaldehyde | |
| Ketone | H3CCOCH3 | Propanane | Acetone | |
| Carboxylic Acid | H3CCO2H | Ethanoic Acid | Acetic acid | |
| Ester | HthreeCCO2CH2CH3 | Ethyl ethanoate | Ethyl acetate | |
| Acid Halide | H3CCOCl | Ethanoyl chloride | Acetyl chloride | |
| Amide | HthreeCCON(CHthree)two | N,Due north-Dimethylethanamide | N,North-Dimethylacetamide | |
| Acid Anhydride | (HthreeCCO)2O | Ethanoic anhydride | Acerb anhydride |
| Return to Tabular array of Contents |
|---|
This page is the belongings of William Reusch. Comments, questions and errors should be sent to whreusch@msu.edu.
These pages are provided to the IOCD to assistance in capacity building in chemical education. 05/05/2013
Source: https://www2.chemistry.msu.edu/faculty/reusch/virttxtjml/intro2.htm
Post a Comment for "what best explains whether bromine (br) or neon (ne) is more likely to form a covalent bond?"